I am please to present a guest post from the illustrious
Dr. Howard Chang of Michigan State University, who presents a perplexing case. Dr. Chang would be interested in reader comments. He writes: "
I can use some help from our colleagues. Any advice
(from anyone) on where and how to proceed for additional studies will be very
much appreciated."
 |
| Dr. Howard Chang |
This is a case of a 12-year-old male with cerebral palsy, severe
developmental delay (level 1-2 years), and seizures (stable, no seizure
episodes since 2 years). He had progressive decline in neurological functions
following flu-like illness. He received IVIG and steroids for clinical
diagnosis of GBS-CIDP (18 months prior to death). Initially he showed some
improvement, but neurological functions continued to decline, with multiple
hospitalizations. MRI imaging studies (2 weeks prior to death) showed
extensive abnormal signal of the cerebral and spinal white matter. He was made
DNR. A general autopsy including brain and spinal cord was performed.
General Autopsy:
1. Atrophy of low
extremity muscles and apparent atrophy of muscles of hands.
2. Cushingoid
appearance with central obesity, skin striations, and adrenocortical atrophy
(likely due to steroid therapy).
Neuropathology Autopsy:
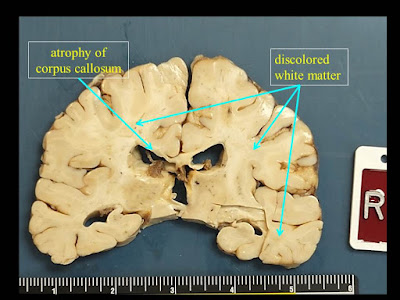
1. Extensive white matter atrophy-degeneration involving both the brain and spinal
cord (leukoencephalomyelopathy) with:
A. Microcephalic brain
(weight 1050 gm, normal should be about 1400 gm).
B. Bilateral cerebral
white matter atrophy-degeneration, with extensive astrogliosis and loss of
axons and myelin affecting the corpus callosum, and multifocal perivenous
microcystic changes involving the centrum semiovale, subcortical white matter,
with focal axonal spheroids in some of the microcystic areas.
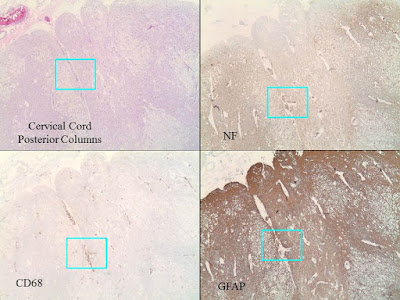
C. Spinal cord with
extensive microcystic degeneration of white matter tracts with loss of axons
and myelin, affecting bilateral posterior, anterior and lateral columns.
Focal loss of neurons within the spinal cord gray matter is noted, including
the anterior horn motor neurons and those in the Clarke’s nuclei. The nerve
roots appear relatively unremarkable.
D. Increased
perivascular macrophages are noted within the brain and spinal cord sections,
but there are no other areas of significant inflammation involving the brain or
spinal cord parenchyma, or the nerve roots. There is no obvious evidence
of abnormal cytoplasmic inclusions within either the neurons or glia.
2. Cerebral infarcts, small, involving the right occipital pole (subacute), and a
lacunar (old) infarct superior to the right occipital horn of the lateral
ventricle.